Sand wasp puzzles: part 1

My latest episode of obsessive research all began with putting a name to this face.
When we met mid January she was unfamiliar to me. A quite common sand wasp, as it turns out, yet largely unknown.
Until now.
Let me introduce Austrogorytes spryi
Perhaps not the most catchy of monikers, but that's not the point. In getting to know her, I've come to know much more about her whole family.
I now recognise her cousins when I see them.
Also, I realise we have three closely related species nesting right here in the forest, right now.
Austrogorytes spryi, A. bellicosus and A. frenchii.
Biodiversity hidden in plain sight.
A puzzle indeed
It turns out I had stumbled upon a rather neglected group of wasps
The genus Austrogorytes is endemic to Australia, so most overseas wasp experts are unfamiliar with it. There are no field guides to Australian sand wasps, unlike the plethora of books covering butterflies, bees, grasshoppers – even cockroaches. In fact, there were virtually no photographic reference points at all. The type specimens for Austrogorytes spryi are held in the British Natural History Museum collection but are not currently available among their online images. And my first go-to points for photo matching ... iNaturalist and ALA ... were both lacking any validated photos for this genus.
So I decided it was a project worth doing. Not that I really needed the added justification or incentive – I do enjoy a challenge.
Solving puzzles is always fun, and I found this one irresistible. Before I could begin, I first had to gather the pieces. They were of all sorts. Yellowed pages from century-old manuscripts, complete with descriptions in Latin. A plethora of insect-specific anatomical terms, their use often changing over time. Two wasps I collected here (and sacrificed) for more detailed investigation.
It took a while and I’ll not go into the full details here. For anyone especially interested in the nitty-gritty, see my workbook notes: A. spryi; A. bellicosus.
The outcome? Not only did I convince myself of the identity of my two specimens (Austrogorytes spryi and Austrogorytes bellicosus). In the process, I learnt to distinguish Austrogorytes from related genera and from each other.
Testing my strategy on a national dataset
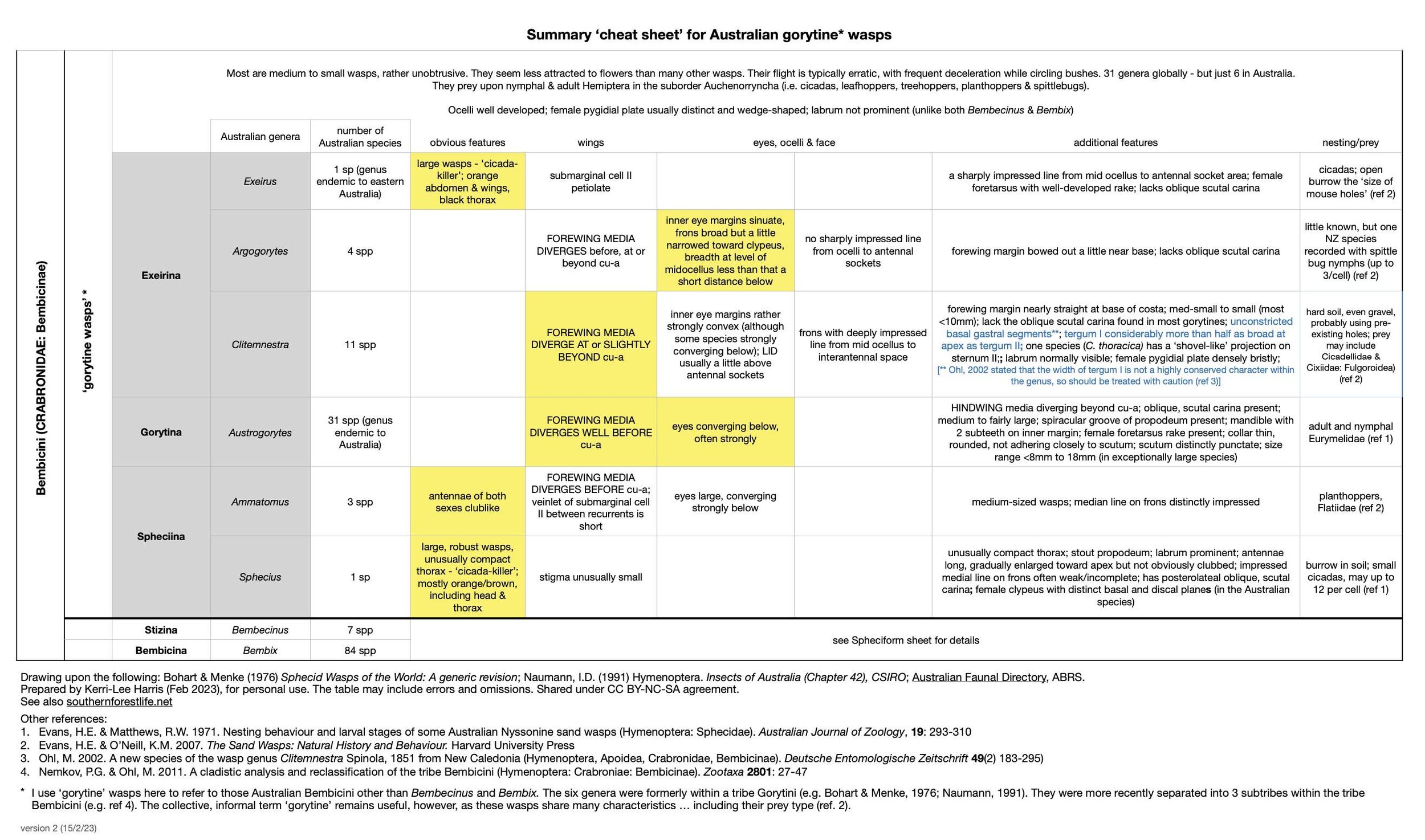
Once I was confident in the identification of A. spryi and A. bellicosus, based on our local wasps, the test was to see if I could apply what I’d learned to a much larger dataset – the nearly 100 sightings of ‘gorytine’ wasps on iNaturalist. I developed a checklist of distinguishing features for each of the 31 species of Austrogorytes, based on a combination of Bohart’s 1984 review and original species descriptions from as far back as 1856. In particular, I focused on the characters most likely to be visible in a photo.
It worked! Nearly all of the sightings online I could take to species level, even where the overall image quality was not great. And here’s what I make of the results:
Austrogorytes spryi is the most commonly sighted species in the genus. Three-quarters of the Austrogorytes observations I found on iNaturalist were of this species.
Most Austrogorytes spryi sightings are of males, no doubt due to their habit of communally roosting on grasses. Clumps of sleepy wasps attract attention.
Austrogorytes spryi is a widespread and variable species – a point made by Bohart (1984).
There was just a handful of sightings of Austrogorytes bellicosus amongst the iNaturalist collection (e.g. Vic, Sydney). This surprised me, as it is first gorytine wasp we recorded here in the forest – although only now do we know its name. In addition, A. bellicosus is much larger than A. spryi, so if they were widespread I would expect to see more observations on iNaturalist. It is the species Howard Evans and Robert Matthews studied near Canberra in the 1970s, and it remains the only Austrogorytes species for which there is published biological information (Evans & Matthews 1971).
The iNaturalist dataset also includes several less common species (well, less commonly photographed, anyway): Austrogorytes frenchii (Vic , & here in our home forest in southern NSW); A. tarsatus (Perth); A. fimbriatus (Perth); A. grahami (Vic, Canberra); and A. chrysozonus (Vic).
Bonus! In disentangling Austrogorytes from related genera, and with a bit more digging through old papers, I was able to identify several related genera … and even call them to species level. For example: Clitemnestra mimetica (Vic); Clitemnestra plomleyi (Vic); Argogorytes secernendus (Vic); and Ammatomus icarioides (Brisbane).
Please note. As with any species identification, I may be wrong. That’s OK. It is the way science works. You give it your best shot, based on the information you have, proffer your evidence and invite constructive critique. I look forward to comments, clarifications and corrections. And as I continue my study of crabronids in general, I may uncover puzzle pieces I overlooked.
It is also worth noting that the taxonomy of Austrogorytes species will probably continue to change over time. New species may be discovered, existing species split, others combined. And who knows what light molecular analyses might cast? Time will tell.
Developing transferable skills (and tools)
Although this exercise began with one wasp, it doesn’t end there. I now have a framework that I can use to tackle crabronid identification more broadly. Notes, strategies and cheat sheets tailored to those features most visible in photographs. Plus, when taking my own photos in the future, I now have a clearer idea of which bits matter most.
Recognising Crabronids
Many wasps can be readily placed to family or subfamily, based on a combination of morphology and behaviour. Spider wasps (Pompilidae), with their long, spiny legs and characteristic hunting behaviour. Paper wasps and potter wasps (Vespidae), with their folded wings, distinctive pronotum and recognisable nests. Cuckoo wasps (Chrysididae), with their thick, often metallic cuticle. Et cetera.
Crabronid recognition is not so straight forward. Crabronidae is a large, diverse family. There is no short list of characters that can be used to define them. Crabronids do not even share a common name - that tends to be left to subfamilies or even tribes.
Indeed, it is even difficult to distinguish a crabronid from a bee – and there's a good reason. Crabronids are more closely related to bees than they are to most wasps (Peters et. al. 2017). The best clue is in their food preferences. Crabronids hunt, bees gather pollen! That only works for the identification of females, but it’s a start.
The antennae sometimes help in distinguishing crabronid wasps from bees. In general, the antennae arise high on the face in bees. In many crabronids - but not all – the antennal sockets are low, close to the clypeus. Not foolproof, but useful nonetheless.
So given the lack of defining features at the family level, how do you know a crabronid when you see one? My approach is ‘if it doesn’t seem a good fit elsewhere, check it against each of the crabronid subfamilies’. Admittedly, my growing familiarity with crabronids helps.
Distinguishing subfamilies and tribes
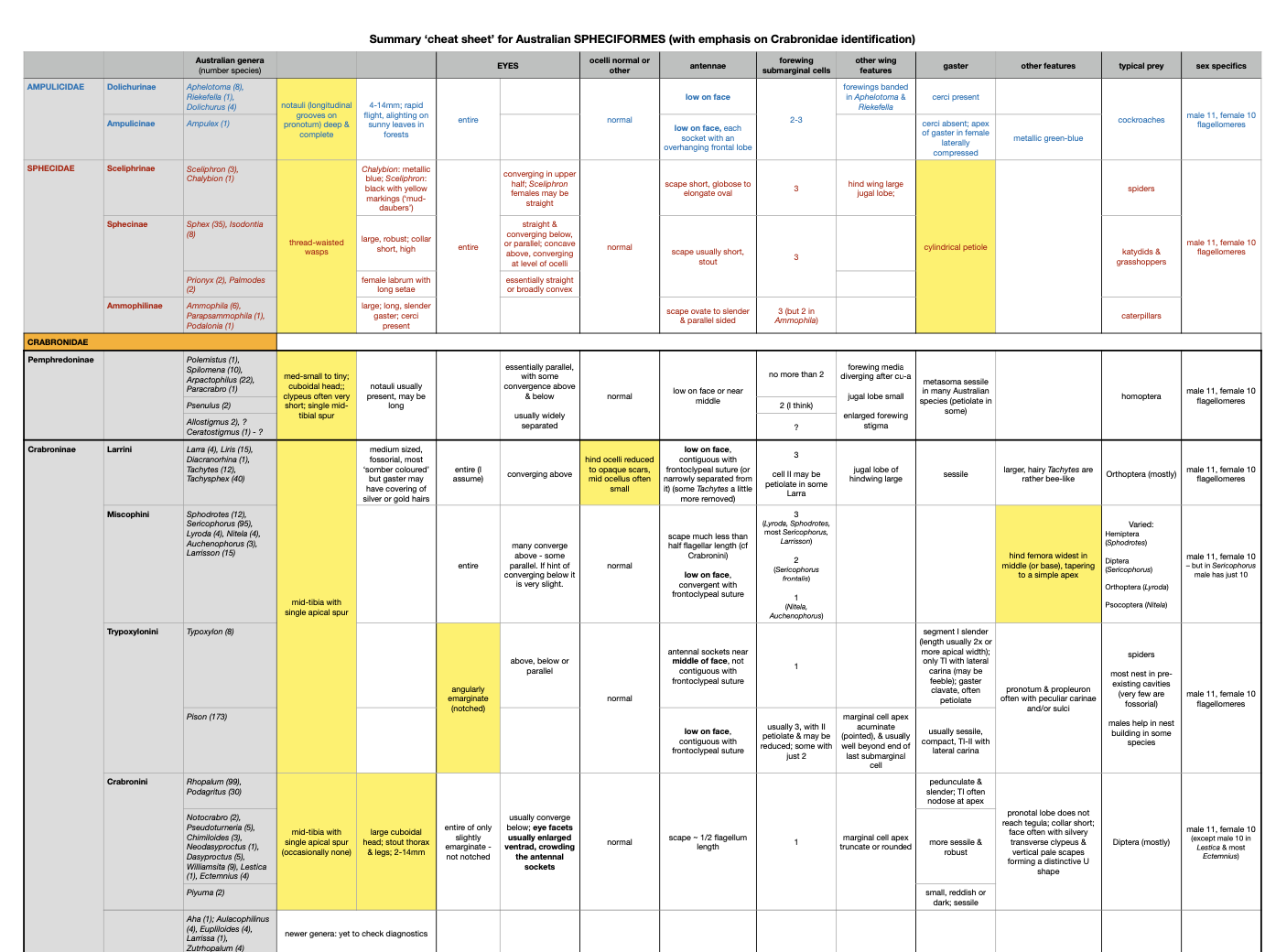
Being methodical (and more than a little obsessive) by nature, I put together a cheat sheet to help me winnow the candidates without overlooking any possibilities. Australia is home to more than 800 crabronid species, across 46 genera. It makes sense to start at the more managable level of the four subfamilies and their 16 tribes and subgroups.
And to help me with Austrogorytes specifically, I also made a cheat sheet for the six ‘gorytine’ genera. That is, the Bembicini other than Bembix and Bembecinus.
When contructing these charts I sought distinguishing features potentially visible in a photo. Our insect ‘collecting’ is nearly all field-based photography. We sometimes bring something into the lab for a closer look, but not often.
The eyes
The eyes provided valuable information for distinguishing between crabronid subfamilies, and in some cases genera. In particular, the shape and alignment of the inner margins of the eyes (from ocelli to clypeus). Fortunately, most of us instinctively try to capture a face shot – and some wasps will deliberately look straight at the bothersome photographer. Win win!
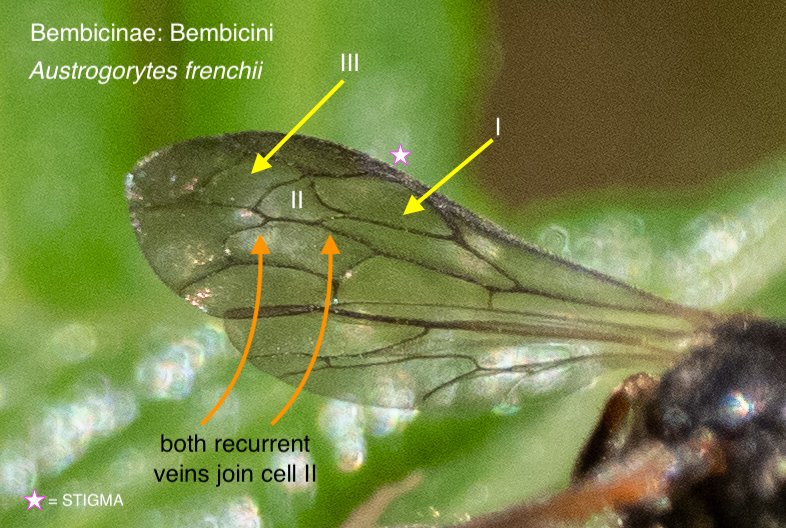
Forewing venation
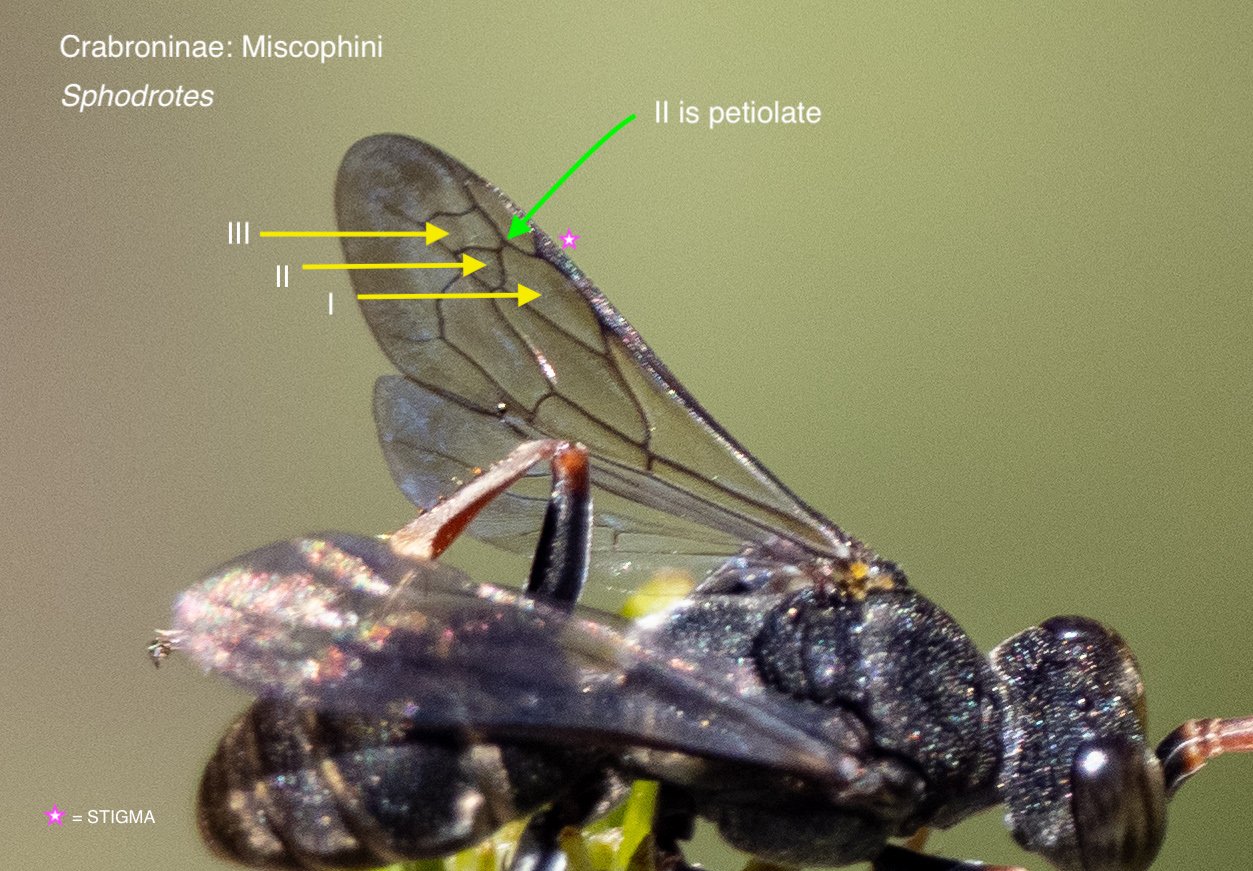
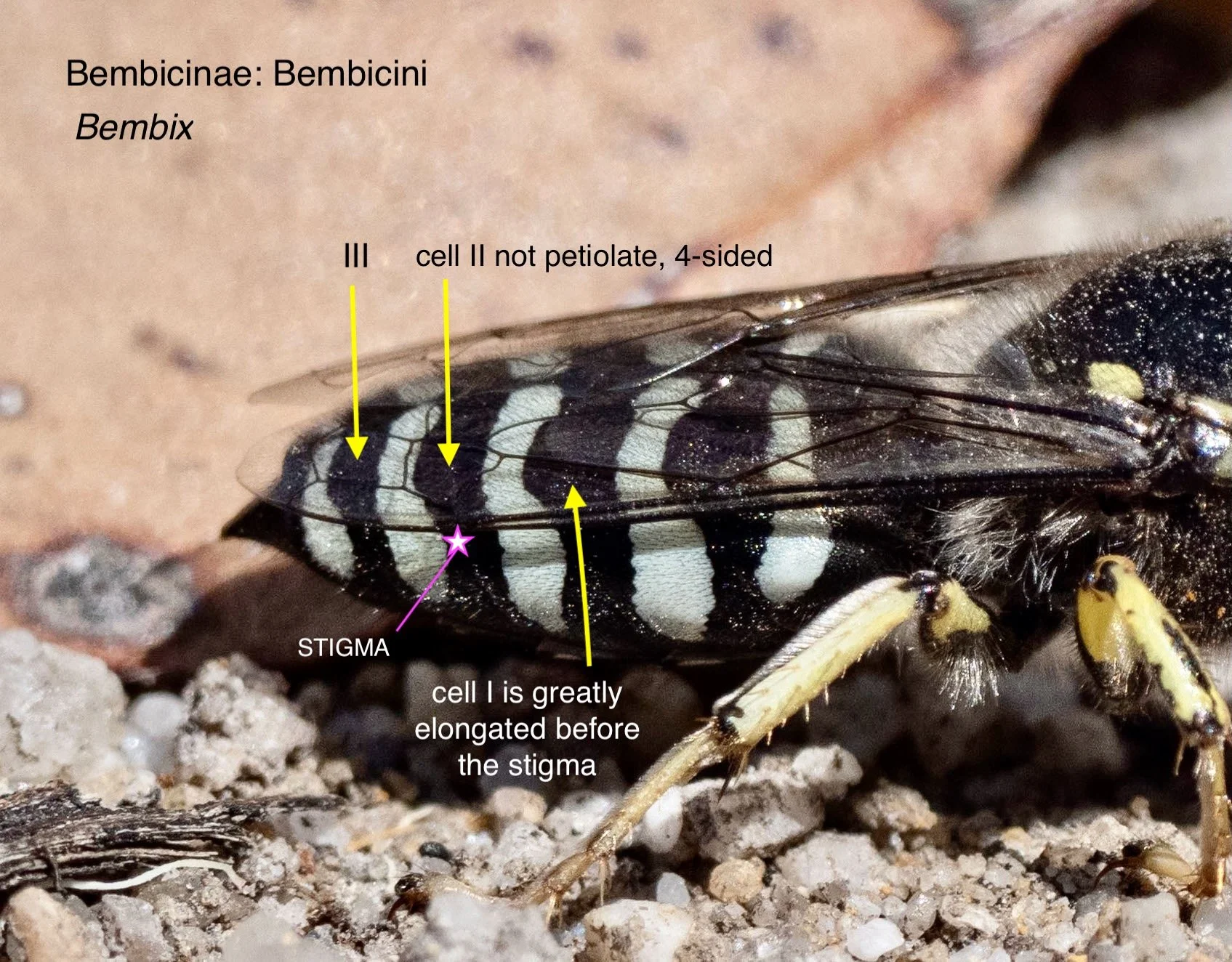
The pattern of veins in the forewing provides crucial information. Luckily, it is relatively easy to capture in a field photograph – particularly when a wasp is at rest and grooming.
The number, size and shape of the submarginal cells helps to distinguish various tribes and subtribes, while the relationship of these cells with the ‘recurrent veins’ can be diagnosic between genera or species.
There is a second forewing feature worth noting: the point at which the media vein diverges with respect to the cu-a crossvein. This feature proved particularly important for distinguishing between Austrogorytes and Clitemnestra. Fortunately, this divergence point (marked in white arrows in the following gallery) is not too difficult to locate even when the wing is viewed obliquely or the image resolution low.
Antennae
For most crabronids, the number of segments in the antennae differs between the sexes. Males typically have 11 flagellar segments while females have 10. The relative length of the scape and its point of attachment to the head is also relevant.
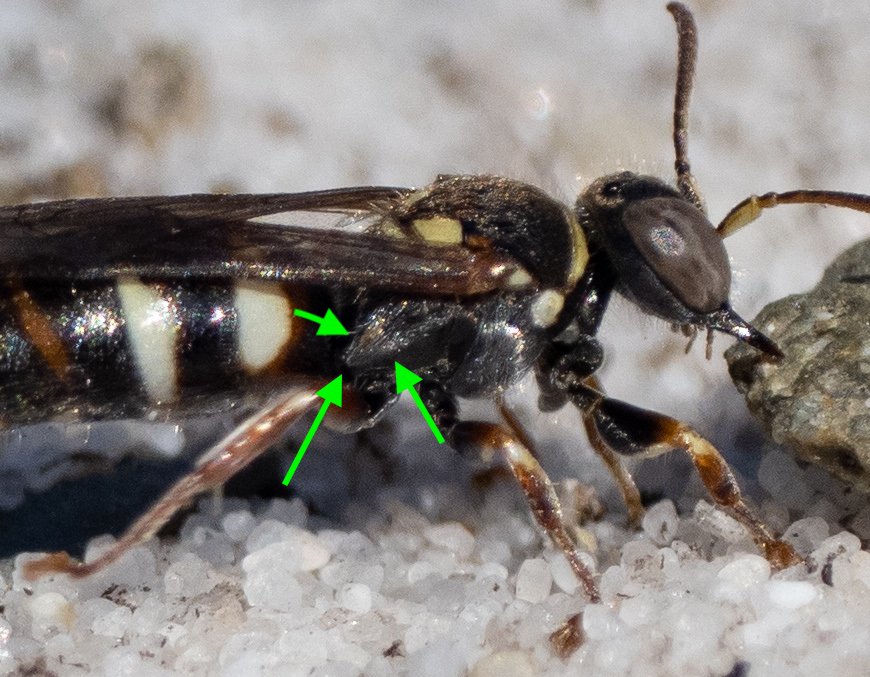
Assorted extras
The following images show other features mentioned in the crabronid sheet. In particular, note the shape of the gaster. The width and shape of the first segment is diagnostic for some taxa.
The best strategy is to take as many photos as possible, from a variety of angles – then hope that it is enough.
Sand wasp puzzles: part 2 … coming soon
My next challenge is Bembix. And it’s a rather large challenge. There are 84 species in Australia and nearly 1000 sightings on iNaturalist. As I did for Austrogorytes, I’ll start by identifying our home species and thereby familiarise myself with the genus. Then I’ll see if I can contribute identifications to at least some of the iNaturalist collection. The one essential piece to the Bembix puzzle is the 1973 monograph by Evans & Matthews – and I’m grateful to have recently received a copy of this precious book. I’ll be putting it to good use in the weeks ahead.
Thanks to all involved in iNaturalist
I have been an iNaturalist enthusiast for a while now. It’s not just the platform’s sophisticated architecture, computer vision (AI), or global reach. The true strength of iNaturalist lies in the collaboration that it fosters between naturalists – novices, keen amateurs and professionals alike. The supportive culture encourages contributions. And the more sightings uploaded and insights offered, the more we can all learn about Australia’s biodiversity.
My recent adventure with Austrogorytes has further convinced me of this. The many unidentified sightings of Austrogorytes, Clitemnestra and related genera proved invaluable. Such a database of photos exists nowhere else. And this resource will continue to grow.
Bohart, R.M. & Menke, A.S. 1976. Sphecid Wasps of the World: A generic revision. University of California Press.
Bohart, R.M. 1984. A revision of the genus Austrogorytes Bohart (Hymenoptera: Sphecidae). Australian Journal of Zoology 32: 391-412
Evans, H.E. & Matthews, R.W. 1971. Nesting behaviour and larval stages of some Australian Nyssonine sand wasps (Hymenoptera: Sphecidae). Australian Journal of Zoology 19: 293-310
Evans, H.E. & Matthews, R.W. 1973. Systematics and nesting behavior of Australian and nesting behavior of Australian Bembix sand wasps (Hymenoptera, Sphecidae). Memoirs of the American Entomological Institute 20: iv, 1-387
Peters, R.S., Krogmann, L., Mayer, C. …. Niehuis, O. 2017. Evolutionary history of the Hymenoptera. Current Biology. 27(7): 1013-1018 (open access … doi.org/10.1016/j.cub.2017.01.027)
Ross, H.E. 1936. The ancestry and wing venation of the Hymenoptera. Annals of The Entomological Society of America 29: 99-111
Turner, R.E. 1915. Notes on fossorial Hymenoptera. XV. New Australian Crabronidae. Annals and Magazine of Natural History 8 15: 62-96